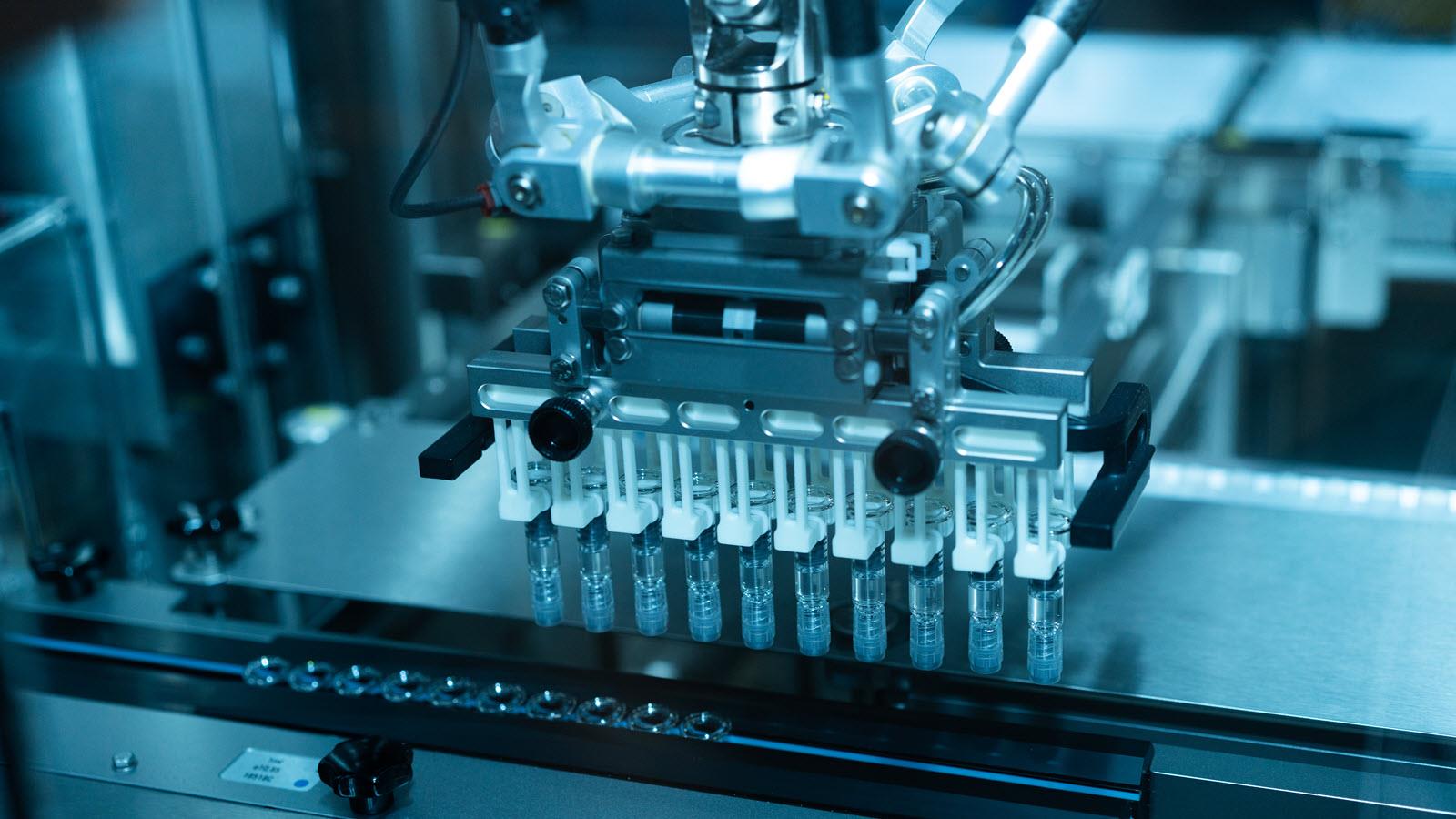
Fill-finish is a critical phase in the manufacture of sterile pharmaceutical products. A typical vaccine requires a vial or syringe. That’s the “fill” part of fill-finish, when vials and syringes are filled with vaccine. Part two is “finish,” when a manufacturer labels, packages and inspects for quality before the product leaves the facility.
This stage of the product lifecycle – including rigorous controls, validated aseptic processes and environmental monitoring – maintains standards for sterility, stability and safety. (“Aseptic” means free of contamination from microorganisms, such as bacteria or viruses.)
“Because fill and finish is the last step before a product reaches the patient, manufacturing must be executed with the highest standards of quality and aseptic practices. Any contamination or deviation at this point can compromise product integrity, patient safety and regulatory compliance,” said Richard Hughes, CSL Seqirus Senior Director of Quality Assurance and Quality Site Head, Holly Springs.
During the COVID-19 pandemic, U.S. manufacturers had to overcome fill-finish bottlenecks. Manufacturers in the United Kingdom experienced the same issue. The U.K. Department of Health and Social Care cited the final stage of filling, inspecting and packing syringes as a common cause of delays throughout the biopharmaceutical industry.
The process takes time, and biopharmaceutical manufacturers that don’t have their own fill-finish operation or lack sufficient capacity must turn to third-party fill-finish sites and wait for an available slot.
How CSL Improves the Fill-Finish Process
Producers of the annual influenza vaccine are well aware of this challenge. To address it, CSL Seqirus added its own fill-finish facility in Merseyside, England, near Liverpool. CSL Seqirus can fill and inspect 600 syringes per minute there. Increased speed and volume in vaccine and pharmaceutical manufacturing strengthen the company’s ability to respond to seasonal influenza and to potential influenza pandemics in the United Kingdom, Australia, and the United States.