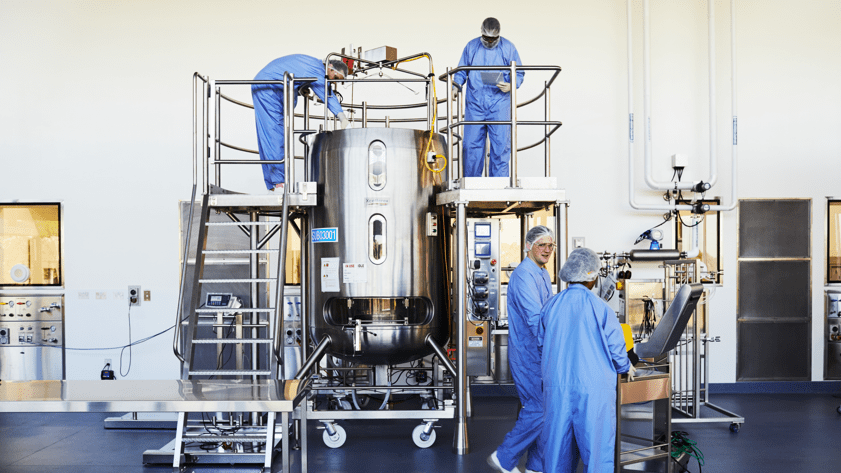
Plasma is a valuable resource for many current and potentially new biological therapies.
CSL relies on our plasma donors to provide this lifesaving resource and as such, we operate under the belief that we have an obligation to maximize the benefit of each donation. The pursuit of state-of-the-art technologies to improve yield and reliability processes for donated plasma continue to be an important, strategic area of focus for CSL therefore we strive to be the industry leader in plasma-derived therapies and increase patient access to these life-changing products. CSL continues to focus on initiatives to increase immunoglobulin (Ig) yield including enhancing the current Ig process robustness and recoveries through data analytics, process optimisation, plasma allocation and operational excellence. In addition to yield, we also focus on new delivery vehicles, new formulations and the development of new plasma medicines to make the most of this natural resource for patients.