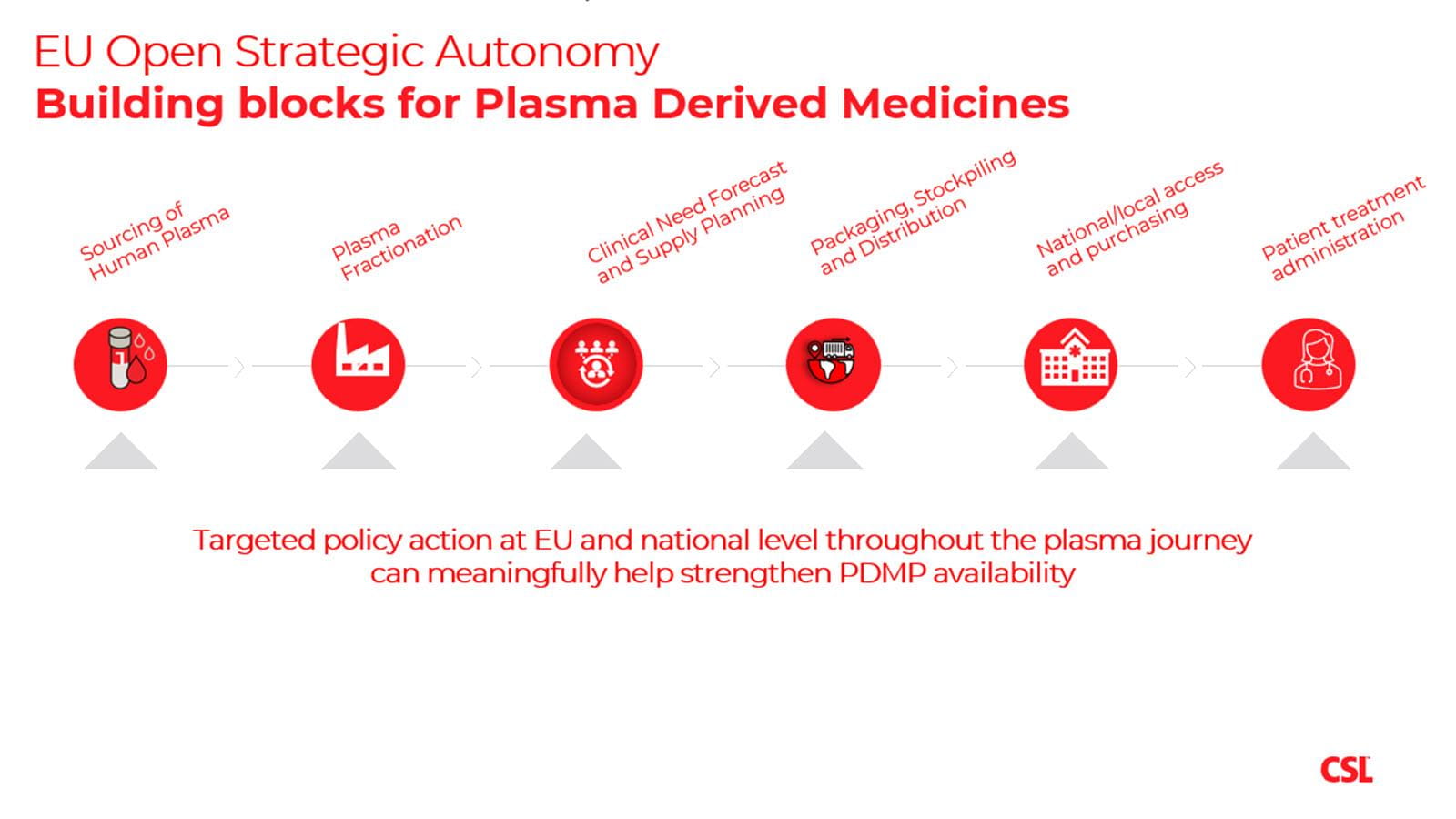
Human plasma contains proteins, such as immunoglobulins and clotting factors, that are turned into medicines for patients who live with rare conditions like primary immune deficiency and hemophilia.
Doing so requires both a steady supply of donated plasma and exacting manufacturing process – two parts of the equation that Europe could improve through policy changes under consideration, CSL Behring shared in sponsored content published on Politico.
Read Open Strategic Autonomy for Plasma Therapies in the EU
The article outlined four imperatives: increase the plasma supply, strengthen the manufacturing base; support a broad availability of therapies; and modernize the supply chain.
“We can meet the needs of EU patients who rely on plasma therapies – and the EU’s goals for strategic autonomy – by ensuring a vibrant plasma-based biotech sector,” according to the article.
Since the Politico article was published, the EU institutions are finalizing several pieces of legislation which may contribute to some of the objectives underlined in the opinion piece. For example, the European Commission has proposed a new Critical Medicines Act, which aims to improve the availability, supply and production of critical medicines within the EU.
CSL Behring is actively engaging in these discussions at a European level, and has been a member of the Critical Medicines Alliance, a consultative mechanism bringing together relevant stakeholders from EU Member States, key industries, the civil society and the scientific community.