CSL Coronavirus Information
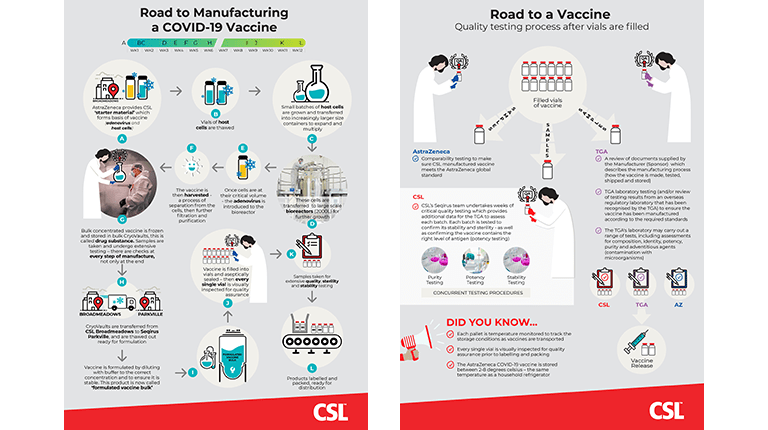
Overview: Manufacturing and Quality Testing a COVID-19 Vaccine at CSL
We've created an infographic to help understanding of the manufacturing and quality testing processes involved in making a COVID-19 vaccine at CSL.
View the COVID-19 Vaccine InfographicNews Releases & Updates
COVID-19 Update
24 JUNE 2022: CSL continues to provide medicines to patients around the world.
CSL’s Global Role in Battling COVID-19
17 SEPTEMBER 2021: Here is how CSL is working around the world with academia, industry and governments to combat the novel coronavirus COVID-19.
CoVIg-19 Plasma Alliance: Topline Results from Trial of Investigational COVID-19 Hyperimmune Globulin Medicine
2 APRIL 2021: Phase 3 Inpatient Treatment with Anti-Coronavirus Immunoglobulin (ITAC) clinical trial did not meet its endpoints to show efficacy in adults hospitalized with COVID-19.
CSL Commences Manufacturing of University of Oxford/AstraZeneca Vaccine Candidate in Melbourne
8 NOVEMBER 2020: CSL has separate contracts with AstraZeneca and the Australian Government to manufacture doses of the AZD1222 vaccine candidate, with first doses planned for release in the first half of 2021, pending the outcome of clinical trials and regulatory approval.
First Patient Enrolled in NIH Phase 3 Trial to Evaluate Potential COVID-19 Hyperimmune Medicine
8 OCTOBER 2020: The anti-COVID-19 Hyperimmune Globulin (CoVIg-19) medicine is under evaluation as part of the trial and may become one of the earliest treatments for hospitalized individuals at risk for serious complications of COVID-19.
The University of Queensland, CEPI and CSL partner to advance development and manufacture of COVID-19 vaccine candidate
5 JUNE 2020: CEPI, the Coalition for Epidemic Preparedness Innovations, CSL (ASX:CSL) and The University of Queensland (UQ) today announced that they have entered into a new, significant partnering agreement to accelerate the development, manufacture and distribution of a COVID-19 vaccine candidate which has been pioneered by researchers at UQ.
In Fight Against COVID-19, CSL Behring Begins Trial to Evaluate Monoclonal Antibody (CSL312) for Respiratory Distress
6 JULY 2020: Phase II placebo-controlled study will assess the safety and efficacy of CSL312 for the treatment of patients with severe respiratory distress due to COVID-19 related pneumonia
Racing Against Time, Medical Researchers, Life Science Companies and COVID-19 Survivors Launch National Campaign to Drive Blood Plasma Donation
26 MAY 2020: “The Fight Is In Us” Campaign Seeks to Mobilize COVID-19 Survivors to Accelerate the Development of Potentially Lifesaving Therapies
CoVIg-19 Plasma Alliance Builds Strong Momentum Through Expanded Membership and Clinical Trial Collaboration
7 MAY 2020: Rapidly expanding support for the Alliance is increasing donations of convalescent plasma to begin clinical production, while NIH collaboration confirms clinical trial approach
CSL Behring Australia Commences Development of Treatment for Serious Cases of COVID-19
6 MAY 2020: Plasma-derived therapeutic with potential to treat serious complications of COVID-19
CSL Behring and SAB Biotherapeutics Join Forces to Deliver New Potential COVID-19 Therapeutic
8 APRIL 2020: COVID-19 treatment candidate, a high-potency immunotherapy delivering human polyclonal antibodies targeted to SARS-CoV-2, generated from SAB’s novel platform technology, on-track for clinical evaluation as early as summer.
Global Plasma Leaders Collaborate to Accelerate Development of Potential COVID-19 Hyperimmune Therapy
6 APRIL 2020: Partnership brings together world-leading plasma companies to focus on developing and delivering a hyperimmune immunoglobulin in the global fight against COVID-19.


